Biological Safety Policy
Purpose
It is the policy of Loughborough University to ensure that all work involving the use of biological material, including biological agents, is subject to the standards of control necessary to prevent, or where this is not possible to minimise, risks to human health, safety and the environment.
Effective biological safety management requires consideration of the safe, responsible, sustainable and economical use of biological material. All aspects of biological material use are governed by a comprehensive set of legislation to ensure the risks posed by substances which may be harmful to human health or to the environment are suitably controlled.
Scope
This policy applies to all work (procurement, handling, use, transportation, storage and disposal) involving biological agents or involving biological material, which could contain biological agents. Biological agents include microorganisms (bacteria, viruses, fungi and microscopic parasites), cell cultures and human endoparasites.
Biological materials are defined as any materials or fluids from or produced by a biological organism. These can include but are not limited to:
- Tissue samples,
- Blood,
- Bone marrow,
- Biopsy samples,
- Environmental samples including food, water, soil, air, sewage and
- Plant material which poses a risk of infection, allergy or toxicity or has a detrimental effect on the wider environment.
The policy applies to:
- All staff, students (both postgraduate and undergraduate) and personnel (e.g. contractors and visitors) at workplaces under the control of Loughborough University.
- All biological agents classified as hazardous under the Control of Substances Hazardous to Health (COSHH) Regulations as defined in the Advisory Committee on Dangerous Pathogens (ACDP) Hazard groups (1-4).
- All work involving biological agents and biological material.
- All genetically modified organisms as classified under the Genetically Modified Organisms (Contained Use) Regulations 2014.
- All animal pathogens covered under Specified Animal Pathogen Order (SAPO) 2008.
- Controlled or prohibited plants, plant products or plant seeds under section 1-6 of the Plant Health Order 2015.
- Biological agents that are covered under Schedule 5 of the Anti-Terrorism, Crime and Security Act 2001.
Please note there are additional policies and procedures for biological material that falls under the Human Tissue Act 2004 and may require ethical approval. Please see the University Human Tissue Authority (HTA) Licence Compliance Quality Manual and University Ethics guidelines for further information. For guidance and best practice surrounding biological agents and biological material, please see Appendix 1 for a list of available guidance documents.
Key Legislative Requirements
COSHH Regulations 2002 and GMO (Contained Use) Regulations 2014
Please refer to Appendix 2 for the key legislative requirements under the COSHH Regulations 2002 and the GMO (Contained Use) Regulations 2014.
Specified Animal Pathogens Order (SAPO) 2008
Under the SAPO 2008 it is prohibited to possess the following without a licence granted by the Secretary of State:
- Any pathogen as listed in Schedule 1 of SAPO, or
- Any carrier in which a known pathogen listed in Schedule is present.
The full list of animal pathogens is listed in Schedule 1 of SAPO. Prior to working with a new animal pathogen, Schedule 1 must be consulted to confirm the classification of the pathogen. If a licence is required, contact the University Health and Safety Service (UHSS).
Plant Health Order 2015
Under the Plant Health Order 2015 it is prohibited to order, receive, or work with any items covered in Schedules 1-6 without a Plant Health Licence. Prior to working with new plant material Schedules 1-6 must be consulted, if a licence is required contact the UHSS.
Anti-Terrorism, Crime and Security Act 2001
Any work with biological agents listed under Schedule 5 of the Anti-Terrorism, Crime and Security Act (ATCSA) 2001 requires notification to the Home Office and visits from a Counter Terrorism Security Advisor (CTSA).
Schedule 5 includes micro-organisms, nucleic acid sequences associated with pathogenicity/or GMOs and toxins. Prior to working with new biological agents, please refer to Schedule 5. If a licence is required contact the UHSS.
The Animal By-Product (Enforcement) (England) Regulations 2013
The Animal By-Product (Enforcement) (England) Regulations 2013 dictate the requirements for the storage, use, handling and transportation of animal by-products (ABPs). The regulations require sites storing or using ABPs to be registered with the Animal and Plant Health Agency (APHA). Prior to working with new ABPs, contact the UHSS.
Duty Holders
The following sections outline the responsibilities of the Duty Holders:
Deans of Schools/Heads of Professional Services
Deans of Schools/Directors/Heads of Professional Services shall:
- Ensure that systems and adequate resources are made available to comply with this policy. In particular:
- To manage the purchasing and acquisition of biological material.
- To implement and maintain effective control measures.
- To establish and maintain inventories of biological materials.
- To conduct inspections of areas where biological material is stored and used.
- To enable training for staff, students and visitors.
- To ensure safe management of waste disposal.
- Facilitate consultation within the School/Service to ensure that arrangements are still effective.
- Appoint person(s) to act as Biological Safety Supervisor to oversee biological safety at a departmental, school or local level as required.
- Authorise the School Safety Officers (SSO)/Department Safety Officers (DSO)/Responsible person (RP) as appropriate to sign Materials Transfer Agreements (MTA) on behalf of the School/Service.
- Ensure all staff, students and visitors have the necessary training and competencies.
- Ensure there are processes for the safe disposal of all biological material.
School and Departmental Safety Officers
In addition to the duties outlined in the Health and Safety policy, SSOs/DSOs shall:
- Where necessary make recommendations to the Dean of School/Directors/Head of Service.
- Ensure that suitable and sufficient risk assessments and standard operating procedures (SOPs) including emergency procedures, are in place and reviewed as necessary.
- Approve biological risk assessments for hazard group 1 and 2 agents.
- Ensure all staff, students, visitors and contractors training is recorded and reviewed as required.
- Verify that plant, equipment and engineering controls are maintained.
- Ensure equipment/work areas are decontaminated and appropriate clearance certificates are issued by the UHSS before transferring to alternative locations or the work area is repurposed.
- Ensure security arrangements are implemented to prevent unauthorised access.
- Ensure that inspection schedules are adhered to and monitor completion of identified actions.
- Advise Line Managers/Academic Supervisors on occupational health referrals regarding health surveillance where appropriate.
Biosafety Supervisors
Biosafety supervisors oversee biological safety at a departmental, school or local level as required.
Biosafety supervisors shall:
- Ensure that biological material is obtained in accordance with procedures and that an inventory is maintained at a school level.
- Approve biological risk assessments for hazard group 1 and 2 agents.
- Ensure risk assessments for work with genetically modified organisms are referred to the Genetic Modification and Biological Safety Committee for approval before work commences.
- Ensure no work is carried out using material that falls out of scope of LU’s licences.
- Ensure safe disposal of all biological material.
- Investigate incidents involving exposure to biological material as requested by the UHSS.
- Oversee and co-ordinate the transfer of biological material to and from external organisations.
- Receive updates from RPs on biological safety matters.
Responsible Person (RP)
RPs are appointed by the Dean of School/Directors/Head of Service to support the SSO/DSOs at a local level.
RPs shall:
- Ensure that all biological material is obtained and stored in accordance with procedures and keep inventories up to date at a local level.
- Ensure suitable personal protective equipment (PPE) is provided and maintained to a good order. Ensure that reusable items are examined for faults before use.
- Co-ordinate the training of all staff, students, visitors and contractors. This training should be recorded and reviewed as required.
- Supervise and ensure that all biological material is disposed of in line with SOPs.
- Report any faults with equipment or facilities to the SSO/DSO and the Estates and Facilities Management (E&FM) as appropriate.
- Follow security arrangements and report breaches of security to the SSO/DSO and Security team as appropriate.
- Follow emergency procedures for dealing with exposures to biological material within their School/Service.
Biological and Genetically Modified Organisms Risk assessors
All activities involving biological material must be risk assessed by a suitably competent individual.
Biological and genetically modified organisms risk assessors shall:
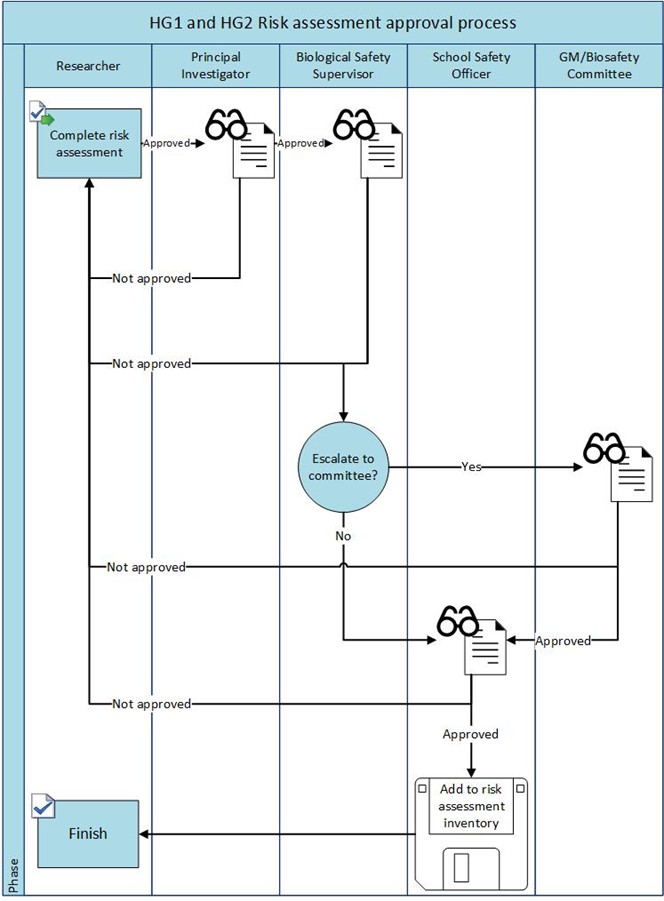
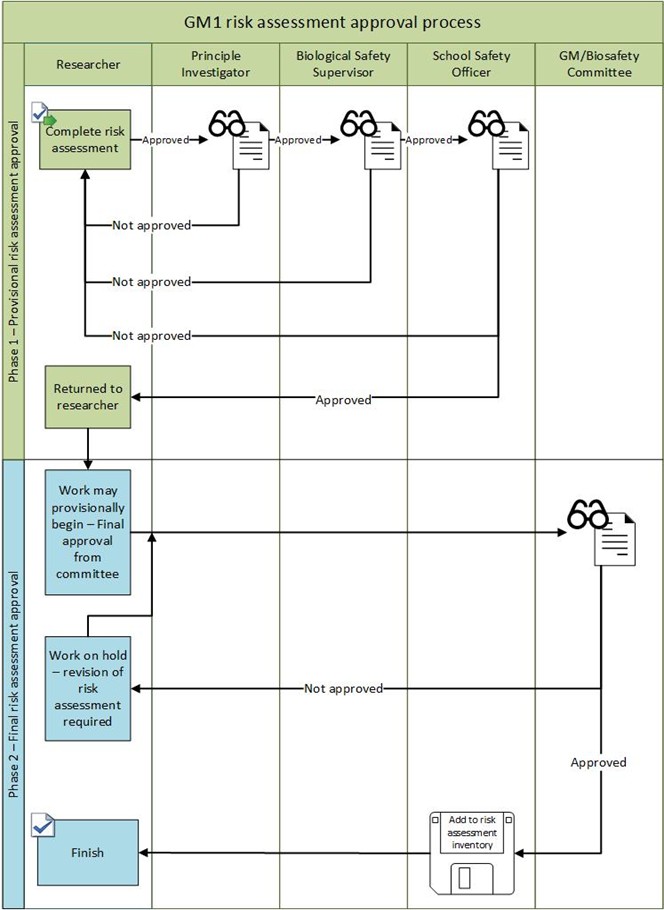
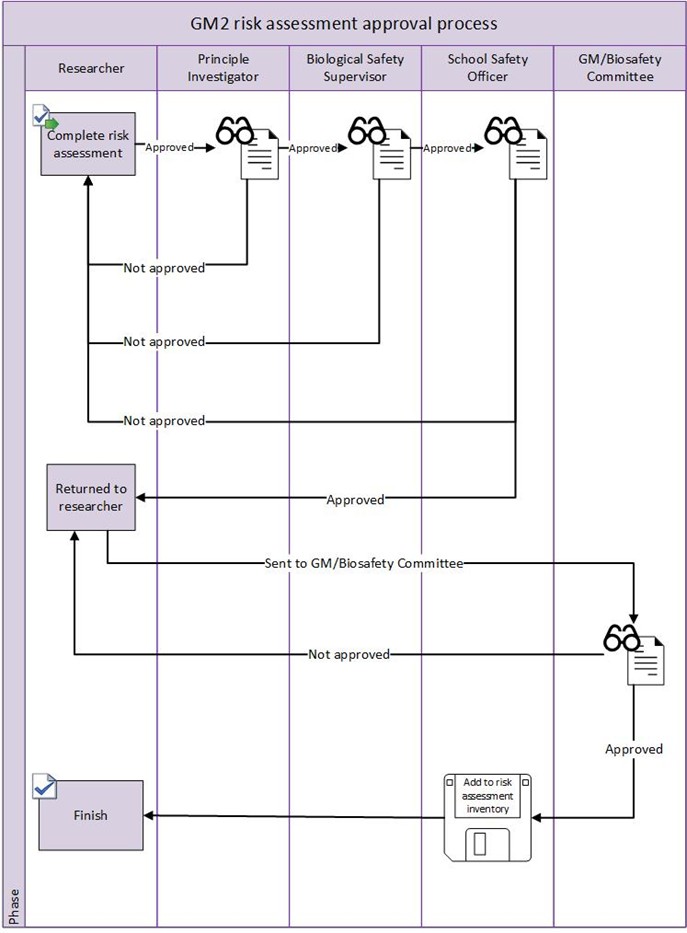
- Carry out suitable and sufficient risk assessments for biological material and genetically modified material. For the risk assessment approval process see Appendices 3 and 4.
- The risk assessment must be recorded and stored in line with School/Service procedures.
The assessor must have adequate and relevant training or experience to ensure they can appropriately assess the risks.
Line Managers, Laboratory Supervisors, and Academic Supervisors
Line managers, laboratory or academic supervisors are responsible for the health and safety of the staff, students and visitors they manage and others who may be affected by their work.
Line managers, laboratory supervisors, and academic supervisors shall:
- Ensure the risks posed by the storage, use, handling and transportation of biological material is assessed and adequately controlled before work commences and that risk assessments are within the agreed review period.
- Ensure personnel they manage/supervise are competent to work with the material and have been provided with sufficient information and training.
- Ensure personnel they manage/supervise follow good laboratory practice and maintain a good standard of housekeeping.
- Ensure that when staff/students they manage leave the School/Service all biological material they were responsible for is either disposed of, or the responsibility for the biological material is transferred to another suitable person.
- Liaise with occupational health service to arrange health surveillance as required.
University Health & Safety Service (UHSS)
The UHSS shall:
- Produce and review the Biological Safety Policy and associated guidance documents.
- Support Deans/Directors/Heads of Professional Services in their duty to provide sufficient training to comply with this policy.
- On request provide information and guidance to staff/students on workplace exposure to biological material.
- Advise E&FM on the design of new laboratories and confirm laboratories meet the standards of their containment level.
- Advise on monitoring for biological material.
- Approve Agresso orders for biological material and genetically modified material.
- Provide clearance certificates for equipment/work areas that have been decontaminated.
- Attend University Genetic Modification and Biological Safety Committee and escalate reports to the University Health, Safety and Environment Committee as necessary.
- Identify key performance indicators (KPIs) and measure performance against them.
- Monitor compliance with this policy through annual biological safety audits.
- Correspond with the Health and Safety Executive (HSE) on operational matters as required.
Genetic Modification and Biological Safety Committee
The Genetic Modification and Biological Safety Committee (GMBC) shall:
- Peer review risk assessments and aid in the classification of genetic modification work.
- Provide comment on policy, guidance documents and protocols involving biological and GMO work.
- Advise the Health, Safety and Environment Committee on biological and GMO matters.
See Appendix 5 for membership and terms of reference.
Environmental Manager
The Environmental Manager shall manage the disposal of clinical and biological material through a central contract, with waste managed locally by the Schools/Services. The procedures are documented as part of Environmental Management System.
Occupational Health Service
The Occupational Health Service shall carry out health surveillance where this is required by risk assessment, or in accordance with schedule 6 of COSHH.
Occupational Health will give advice on health surveillance requirements and provide appropriate vaccination to individuals working with biological agents in line with biological and GMO risk assessments.
Estates and Facilities Management
- Manage a register of items that have been installed following a biological and genetic modification risk assessment whenever this apparatus forms part of the University estate.
- Ensure that items on the register are thoroughly inspected and tested at a frequency not less than stipulated in the relevant legislation.
- Report the results of the inspection/testing to suitable School/Service representative and instigate remedial action where necessary.
- Provide technical advice to Schools/Services on plant, equipment and engineering controls managed by E&FM.
- Keep records of inspection/testing for at least 3 years.
Employees, Students and Visitors
Employees, students, and visitors shall:
- Attend training as requested.
- Work in accordance with risk assessments and SOPs to ensure the health and safety of themselves and others when working with biological material.
- Report any equipment defects, unsafe conditions, near misses and accidents.
Contractors
Contractors shall:
- Co-ordinate with the School/Service representative and follow instructions while on site.
- Ensure their work does not disrupt the work of staff and students and if necessary, ensure their work area is controlled.
- Report any issues to the School/Service representative.
General Requirements and Guidance
Purchase and Acquisition
Schools/Services must have a procedure in place to manage the authorisation, purchase, acquisition, recording and receipt of biological material in line with the relevant legislation. Schools/Services should ensure biological materials are only procured from reputable sources such as established suppliers, other higher education institutions and research facilities.
All biological material and agents procured or gifted into the university which are to be used for research purposes must be accompanied by a Material Transfer Agreement (MTA). Contact the Research Officer for more information on MTAs and see section 4.5 for more information on the transport and transfer of biological materials.
Currently only hazard groups 1 and 2, and GM classes 1 and 2 materials can be purchased.
Inventory, Labelling and Storage
Schools/Services must maintain an inventory of biological material. This inventory must be electronic, backed up and secure.
Storage containers, such as freezers, fridges or cryogenic tanks, should be labelled with biohazard labels and secured.
Inventory, Labelling and Storage
The UHSS shall conduct annual audits of areas using biological material. Trends across audits will be reported to the GMBC and the University’s Health, Safety and Environment committee.
Schools/Services must conduct inspections at least every six months of areas using biological material. Please see the Code of Practice for Biological Laboratories for further information on inspections.
Risk Assessments
For risk assessment requirements under COSHH Regulations 2002 and GMO (Contained Use) 2014, see Appendix 2. For the risk assessment approval processes see Appendices 3 and 4.
Transport and transfer of biological materials
Biological material should be transported in a safe manner to avoid or reduce the risk of spillage and contamination. The route of travel should minimise contact with communal areas as far as possible.
Staff or students wishing to transfer or receive biological material to/from another organisation must first contact their biosafety supervisor and arrange a formal Materials Transfer Agreement (MTA) and contact the Research Office for further details.
Disposal and Decontamination
Schools/Services must consider the waste disposal route before purchasing or using biological material as part of the risk assessment. All biological material must be deactivated prior to disposal.
All equipment which has been used in conjunction with biological material must be decontaminated and assessed for any residual risk posed before it is released for maintenance, repair or disposal. Please speak to the UHSS for clearance certificates, including extractions clearance certificates.
Under GMO (CU) regulations, all contaminated waste as a result of class 2, 3 and 4 work must be inactivated by a validated means prior to disposal. This is not required for class 1 work so long as the GMO:
- does not have the potential to cause harm to human health or the environment,
- is biologically contained (e.g. possess multiple disabling mutations or restrictive nutrient requirements that cannot be met outside the laboratory),
- does not have the capacity to establish and multiply in the environment, and
- does not have capacity to transfer genetic material to other micro-organisms (eg non-mobilisable plasmid).
Please see the guidance document on Disposal of Laboratory Waste for further information.
Emergency Arrangements
Emergency procedures and arrangements must be documented in the risk assessment. Schools/Services must have SOPs for foreseeable emergencies for the activities they are conducting. Please see Appendices 6 and 7 for flowcharts on sharps injuries and spillages.
Where there is serious risk to health, immediate steps should be taken to mitigate the effects, and provide information to those who may be affected.
Accidents, incidents and near-misses must be reported using the University’s incident reporting system.
Serious incidents involving GMOs are reportable to the HSE, it is the duty of the UHSS to report these incidents. These include the loss of containment for GMOs which result in a significant and unintended release of the GMO which presents an immediate or delayed risk to human health or the environment.
Training, Instruction and Supervision
All staff and students must have suitable instruction and training to enable them to work with biological material safely.
An appropriate level of supervision must be determined by risk assessment.
Training needs must be reviewed on a regular basis or when there are significant changes to the work.
Further information on training can be found in the Code of Practice for Biological Laboratories.
Vulnerable groups
There are individuals who are more susceptible to ill health than others and so are more vulnerable than the general population. Vulnerable groups include immunocompromised individuals, such as those who have recently undergone chemotherapy or other cancer treatments, disabled workers, those with underlying health conditions, and young workers/students.
New and expectant mothers are at an additional risk when working with biological material as certain infections can be transmitted from the mother to the developing foetus/newborn infant. During pregnancy, the infection can be transmitted across the placenta. Infections can be transmitted from the mother to the newborn infant when breast feeding or having close physical contact.
Biological agents that can affect the developing foetus include but are not limited to:
- Chlamydia abortus,
- Listeria monocytogenes,
- Parvovirus,
- Cytomegalovirus,
- Rubella virus,
- Blood borne viruses such as HIV, Hepatitis B, C, A, and E,
- Toxoplasma gondii and
- Varicella-zoster virus.
It is the responsibility of line managers/supervisors to review work activities with the individual once they are informed of the pregnancy to ensure a new and expectant mothers risk assessment is carried out, and any additional control measures are identified and implemented.
Appendix 1 – Guidance Documents
The following documents are available on the Biological Safety page on Loughborough University’s website:
Codes of Practice
Appendix 2 – Key Legislative Requirements
Control of Substances Hazardous to Health Regulations (COSHH) 2002
|
Regulation |
Duty |
|
6 – Risk assessment |
|
|
7 – Prevention or control |
To consider and control for the following when reviewing hazards:
|
|
8 – Use of control measures |
|
|
9 – Maintenance |
|
|
10 – Monitoring exposure |
|
|
11 – Health surveillance |
Once an employee is found to have an identifiable disease or adverse health effect, the employer must notify:
|
|
12 – Information, instruction, and training |
|
|
13 - Emergency provisions |
|
|
14 – Fumigations |
|
|
Schedule 3 – Part 5 |
The HSE must be notified of the first use of group 3 and group 4 agents and of the use of the following group 2 agents:
|
Genetically Modified Organisms (Contained Use) Regulations 2014
|
Regulation |
Duty |
|
5 – Risk assessments for micro-organisms And 6 – Risk assessments for larger GMOs |
|
|
7 - Review and recording of risk assessments |
|
|
8 – Advice from a genetic modification safety committee |
|
|
18 – Principles of Occupational and environmental safety |
|
|
19 – Containment and control measures for micro-organisms |
|
|
20 – Containment and control measures for larger GMOs |
|
|
21 – Emergency plans |
|
|
22 – Information relating to accidents |
If an accident occurs, the competent authority of the accident must be informed and told:
|
Appendix 3 – Biological Risk Assessment Sign off flowchart
Appendix 4 – GM Risk Assessment Sign off flowcharts
Appendix 5 – GMBC
Purpose
The purpose of the committee is to comply with the GMO (CU) regulations requirement for a genetic modification safety committee to approve risk assessments for GM class 2 activities and above.
Committee Membership
Membership of the GMBC consists of:
- Chair
- Designated Individual (DI) of the HTA licence
Representation from
- Estates and Facilities Services Management
- School of Aeronautical, Automotive, Chemical and Materials Engineering
- School of Architecture, Building and Civil Engineering
- School of Design and Creative Arts
- School of Science
- School of Sport, Exercise and Health Sciences
- STEMlab
- University Health and Safety Service
- Wolfson School
- Secretary
Optional Attendees:
- Director of Health and Safety Service
- Environmental Manager
- Representation from Occupational Health Service
- Local Exhaust Ventilation (LEV) Duty Assigned Person (DAP)
Terms of Reference
- Provide a forum for discussion and collaboration on best practice surround biological safety and working with GMOs.
- Peer review risk assessments and aid in the classification of genetic modification work.
- Provide comment on policy, guidance documents and protocols involving biological and GMO work.
- Review incident trends and patterns involving biological and GMO work and share lessons learnt.
- Review trends in biological safety audits and share best practice.
- Receive feedback from the HTA committee via the DI on matters relating to biological safety.
- Advise the Health, Safety and Environment Committee on biological and GMO matters.
- Review these terms of reference every three years, or if significant changes occur.
Fixed Agenda Items
- Minutes/Actions from previous meeting
- Matters arising from the minutes
- Update from the University Health and Safety Service
-
- Regulatory updates
- Strategic updates
- Central H&S updates
- Trends in audit results
- Trends and lessons learned from incidents, accidents and near-misses
-
- Updates from Schools and Services
- GM Risk assessments
- AOB
Frequency of Meetings
The GM/Biological Safety Committee will meet three times a year. Additional meetings will be held if required.
Risk assessment approval process
GM risk assessments should be sent to the committee for feedback. Committee members will be given two weeks to provide feedback on the risk assessment. The UHSS will review all comments and suggest to the committee that the risk assessment should be:
- Approved and a signed copy sent to the originator.
- Returned to the originator with request for further information and/or minor changes.
- Returned to the originator with major changes.
The originator can re-submit a risk assessment for approval once changes have been implemented.