Research
Phosphate glasses for biomedical applications
Phosphate glasses dissolve harmlessly when implanted into the body, at a rate which depends very sensitively on the composition of the glass. This makes them applicable as delivery devices for drugs, nutrients, or antimicrobials, where these need to be delivered at a controlled rate. Over the past few years, we have used computer simulation to understand the structural features of the glass which control the dissolutions, and the effects on the dissolution of including therapeutically useful molecules.
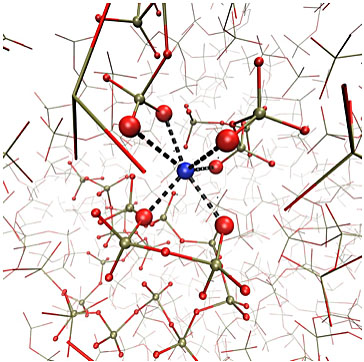 At biomedically relevant compositions, the atomic structure of the glass is made up of short chains of phosphate tetrahedra, which are chemically bonded to sodium and calcium atoms. We showed that the dissolution rate depends very strongly on this bonding, and that by varying the concentration of sodium or calcium atoms in the glass, control of the dissolution rate can be achieved. We have extended this work to consider the effects of incorporating other ions into the glass including silver, which is an antibiotic, strontium, which is a treatment for osteoporosis, and fluorine for dental applications.
At biomedically relevant compositions, the atomic structure of the glass is made up of short chains of phosphate tetrahedra, which are chemically bonded to sodium and calcium atoms. We showed that the dissolution rate depends very strongly on this bonding, and that by varying the concentration of sodium or calcium atoms in the glass, control of the dissolution rate can be achieved. We have extended this work to consider the effects of incorporating other ions into the glass including silver, which is an antibiotic, strontium, which is a treatment for osteoporosis, and fluorine for dental applications.
