Fuel cells and electrolysers
This research investigates a new material, LSAZ, that makes solid oxide fuel cells (SOFCs) work better at lower temperatures, boosting their efficiency. It does this by using a special structure with an amorphous surface layer that conducts ions well due to defects in the material, paving the way for more efficient and eco-friendly SOFCs.
Due to a rise in advanced green energy conversion devices, the transport of ions within solid oxide electrolytes is a fundamental process. These electrolytes are key components of devices like solid oxide fuel cells which have the potential to revolutionise clean energy production.
However, a longstanding challenge has been the need for extremely high operational temperatures, typically exceeding 700°C. These high temperatures are necessary to ensure significant bulk and grain boundary diffusion of ions to enable sufficient ionic conductivity for efficient fuel cell operation.
To address the high-temperature challenge, this study introduces an innovative material known as La0·8Sr0·2Al0·8Zn0·2O3-δ (LSAZ). LSAZ can boost the mobility of ions at a relatively low temperature of around 450 to 550°C for solid oxide fuel cells (SOFCs). The significance of LSAZ showcases the potential to revolutionise SOFC technology and reduce the energy input required for advanced energy conversion devices.
.jpg)
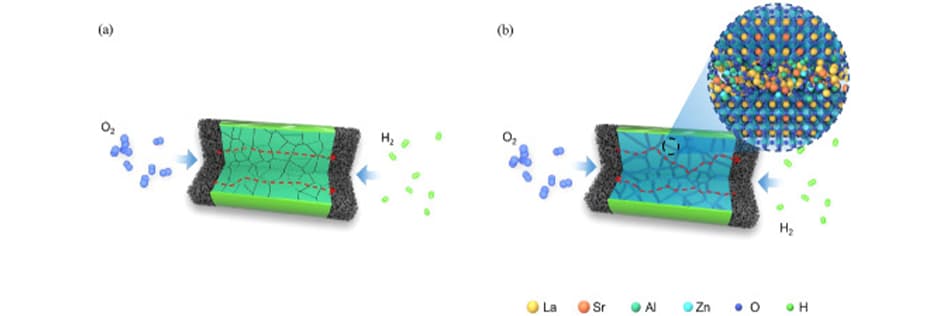
Using LSAZ for ionic conductivity
The LSAZ heterostructure includes an insulating perovskite core and a superionic-conducting amorphous surface layer. This dual nature of LSAZ enables efficient ion transport at lower temperatures. The insulating core provides a stable structure, while the supericonic-conducting surface layer allows for rapid ion movement.
The fuel cells utilising LSAZ demonstrate remarkable results, with power generation reaching 1296 mW cm−2 at 550°C. To put this into perspective, this performance level is approximately 300 times better than that achieved with traditional SOFCs using LSAZ processed at higher temperatures.
One of the key factors contributing to LSAZ's exceptional performance is the presence of oxygen vacancy defects in its amorphous surface layer. These defects play a crucial role in facilitating efficient ionic conduction. This discovery sheds light on the importance of material structure and defect engineering in enhancing ion transport for green energy technologies.
This breakthrough opens up new possibilities for developing highly conductive electrolytes for solid oxide fuel cells. These advanced materials could operate at significantly lower temperatures, making SOFCs more energy-efficient, environmentally friendly, and accessible for various applications in the field of green energy.
References
Xu, D, Yan, A, Yang, Y, Xu, S, Zhou, Y, Yang, S, Lin, W-F (2023) Fast ion-conductive electrolyte based on a doped LaAlO3 with an amorphous surface layer for low-temperature solid oxide fuel cells, Journal of Power Sources, 561, 232723, ISSN: 0378-7753. DOI: 10.1016/j.jpowsour.2023.232723.
Xing, Y, Zhu, B, Hong, L, Xia, C, Wang, B, Wu, Y, Cai, H, Rauf, S, Huang, J, Asghar, MI, Yang, Y, Lin, W-F (2022) Designing High Interfacial Conduction beyond Bulk via Engineering the Semiconductor–Ionic Heterostructure CeO2−δ/BaZr0.8Y0.2O3 for Superior Proton Conductive Fuel Cell and Water Electrolysis Applications, ACS Applied Energy Materials, ISSN: 2574-0962. DOI: 10.1021/acsaem.2c02995.