Enabling catalysts for green energy
This research explores 'Ni5P4' as an efficient and stable electrocatalyst for green hydrogen production in water splitting, using a blend of experimental and theoretical methods to see activity and electronic structure interactions.
In a world increasingly focused on clean energy solutions, the production of green hydrogen is a promising avenue. Green hydrogen, created through water splitting using renewable energy sources, offers a sustainable and eco-friendly energy carrier for the future. However, a significant challenge lies in finding cost-effective and efficient catalysts to speed up the process.
Recent research has uncovered a potential solution in the form of a low-cost material, Ni5P4. Our research explores the material's electrocatalytic properties for the hydrogen evolution reaction, shedding light on why it works so well and how it could contribute to the widespread adoption of green hydrogen production.
The importance of hydrogen in a net zero world
Hydrogen can serve as the primary energy carrier of future energy systems due to its several distinct advantages such as high energy density and zero-carbon emission. Electrocatalytic water splitting is an environmentally friendly way to produce green hydrogen by consuming the electricity generated from intermittent renewable energy sources such as wind, hydro, tidal and solar energy.
Hydrogen Evolution Reaction (HER) is an electrochemical half-reaction of water splitting. It requires high-performance electrocatalysts to proceed expeditiously, particularly under alkaline media where HER is more difficult but Oxygen Evolution Reaction (OER, the other half-cell reaction in water splitting) is much more facile than those in acidic media.
It has been recognised that noble metals exhibit an excellent capability of efficiently catalysing the HER. However, their high cost and low earth abundance limit their application in large-scale green hydrogen production. Therefore, it is essential to develop low-cost high-performance HER electrocatalysts.
.jpg)
Exploring a novel material as an electrocatalyst
Recently, a low-cost Ni5P4 material has been demonstrated experimentally and theoretically to exhibit excellent electrocatalytic activity toward HER. However, a fundamental understanding of the origin of Ni5P4(0001) activity is still lacking. In this work, density functional theory (DFT) calculations were employed for a comprehensive investigation.
The mechanisms and factors behind Ni5P4's activity include:
- Surface structure and hydrogen adsorption
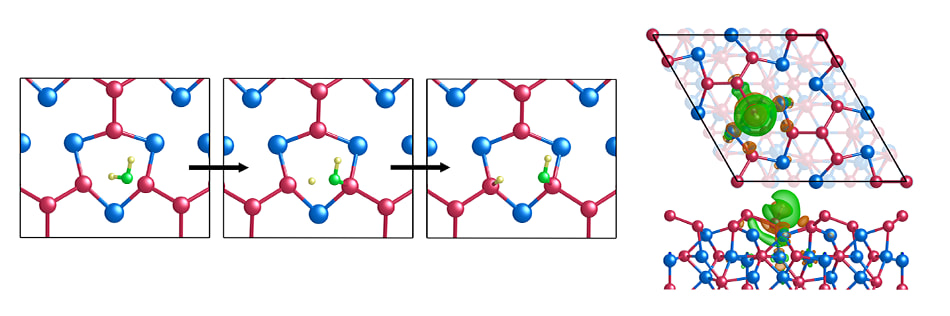
The calculation results indicate that the Ni5P4(0001) surface exposing Ni3P4 termination gains the highest stability, on which nearly thermoneutral hydrogen adsorption was found at the P3-hollow sites, providing a high activity for HER. The activity was also observed to be maintained over a wide H-coverage. - Reaction mechanism
HER occurs via the Volmer-Heyrovsky mechanism as evidenced by the optimal hydrogen adsorption-free energy, but unlikely through the Tafel reaction due to its large energy barrier. Furthermore, the P3-hollow sites also exhibit a low kinetic barrier for water dissociation, promoting HER in alkaline media. - Electronic structure analysis
A series of electronic structure analyses were performed to gain insights into the origin of the HER activity. First, the density of states (DOS) and crystal orbital Hamilton population (COHP) analyses revealed a favourable interaction of electronic states between P and H atoms, leading to stable H adsorption at P3-hollow sites. In addition, the Bader charge analysis demonstrates that the strength of H adsorption at P3-hollow sites linearly increases with the electrons carried by the latter. The optimal net charge on the P3-hollow sites leads to a desired ΔGH that is close to zero. Finally, a highly efficient electron transfer was observed between the P3-hollow sites and their neighbouring atoms, facilitating the HER.
References
Yang, Y, Lin, X, Li, Y, Sheng, T, Cheng, S, Sun, X, Lin, W-F (2023) Insights into the Origin of High Activity of Ni5P4(0001) for Hydrogen Evolution Reaction, The Journal of Physical Chemistry C, ISSN: 1932-7447. DOI: 10.1021/acs.jpcc.3c00238.
Chen, L-N, Wang, S-H, Zhang, P-Y, Chen, Z-X, Lin, X, Yang, H-J, Sheng, T, Lin, W-F, Tian, N, Sun, S-G, Zhou, Z-Y (2021) Ru nanoparticles supported on partially reduced TiO2 as highly efficient catalyst for hydrogen evolution, Nano Energy, 88, pp.106211-106211, ISSN: 2211-2855. DOI: 10.1016/j.nanoen.2021.106211.
Zhou, D, Li, P, Lin, X, McKinley, A, Kuang, Y, Liu, W, Lin, W-F, Sun, X, Duan, X (2021) Layered double hydroxide-based electrocatalysts for the oxygen evolution reaction: identification and tailoring of active sites, and superaerophobic nanoarray electrode assembly, Chemical Society Reviews, 50, pp.8790-8817, ISSN: 0306-0012. DOI: 10.1039/d1cs00186h.