A new generation of modules is emerging made using a chemical compound known as cadmium telluride (CdTe) and over 25 GW has already been installed.
Solar panel efficiency refers to a panel’s ability to convert energy from the sun into electrical energy.
Experts are looking to increase the efficiency of CdTe modules as it has the potential to be competitive on efficiency but at a lower manufacturing cost than silicon-based modules, which use mono-crystalline silicon (c-Si).
The carbon footprint of CdTe is also half that of c-Si modules and end-of-life module recycling for CdTe modules is guaranteed.
Untreated CdTe has very low efficiency and is typically about only 1%. However, when CdTe undergoes a chlorine treatment – which involves treating CdTe with cadmium chloride at 420oC for 20 minutes – its efficiency jumps dramatically. The record cell efficiency is 22%.
Until now, how or why chlorine improves efficiency so drastically was not fully understood.
For the first time, Loughborough University's Dr Pooja Goddard and Professor Roger Smith, along with Dr Peter Hatton and Dr Michael Watts (PhD graduates of Loughborough), have modelled the mechanism by which chlorine improves the efficiency of CdTe.
The study is a joint effort with Professor Mike Walls’ experimental research group housed at Loughborough’s Centre for Renewable Energy Systems Technology (CREST).
It is hoped the findings, published today in Nature Communications, will improve understanding of how chlorine enhances electrical performance and lead to further tuning, resulting in even higher efficiencies (>25%). This would help CdTe solar modules to produce even lower cost electricity.
Research findings and the missing piece
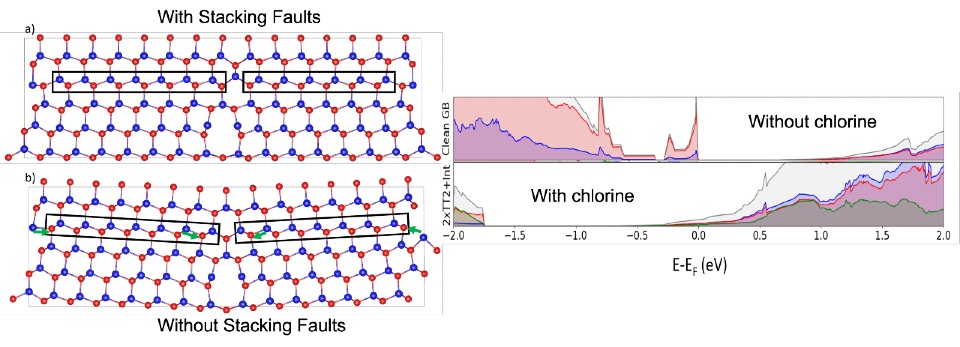
When trying to understand why chlorine improves efficiency, the main observation from previous studies was that defects – known as ‘stacking faults’ – were removed after chlorine treatment.
For a long time, it was believed that stacking fault removal was responsible for the efficiency increase. However, theoretical calculations by the Loughborough group showed that the stacking faults had no effect on cell efficiency.
Previous research from the team showed that instead, it is regions of the material called ‘grain boundaries’, where crystals of different orientation join together, that are responsible for the poor cell efficiency.
In their latest study, Dr Goddard‘s group used quantum mechanical methods to understand the role of chlorine in both improving efficiency and also in removing stacking faults.
Grain boundaries are very complex and full of defects that can act as traps for electrons (subatomic particles that act as the primary carrier of electricity in solids), making these areas ‘active’.
In a process known as ‘passivation’, chlorine is able to deactivate some of the traps and make the grain boundaries less active – therefore increasing the efficiency of CdTe.
The missing piece was understanding how the stacking faults disappear.
The new paper shows that if there is enough chlorine in the grain boundaries, a cascade mechanism is triggered that structurally removes the stacking faults.