Case study 1

Battery-electrolysers
This is an example of work within the team looking at low-cost production of hydrogen through a battery-electrolyser (similar functionality as the Delft-developed battolyser but using different chemistries). It starts life as a battery or flow battery that can produce hydrogen under electrolysis. The water consumption of this reaction is compensated by adding water to the electrolyte to avoid a concentration increase of the electrolyte that would subsequently affect the battery-electrolyser's chemistry.
The battery-electrolyser works by:
- Discharging as a battery when electricity demand exceeds generation
- Charging as a battery
- Use excess electricity to produce hydrogen
A battery-electrolyser was first developed at Delft University by Dr. Fokko Mulder et al. based on Nickel-Iron batteries. Loughborough has been investigating alternative battery-electrolyser chemistry solutions using more abundant materials.
We are looking at two different use cases, as shown below.
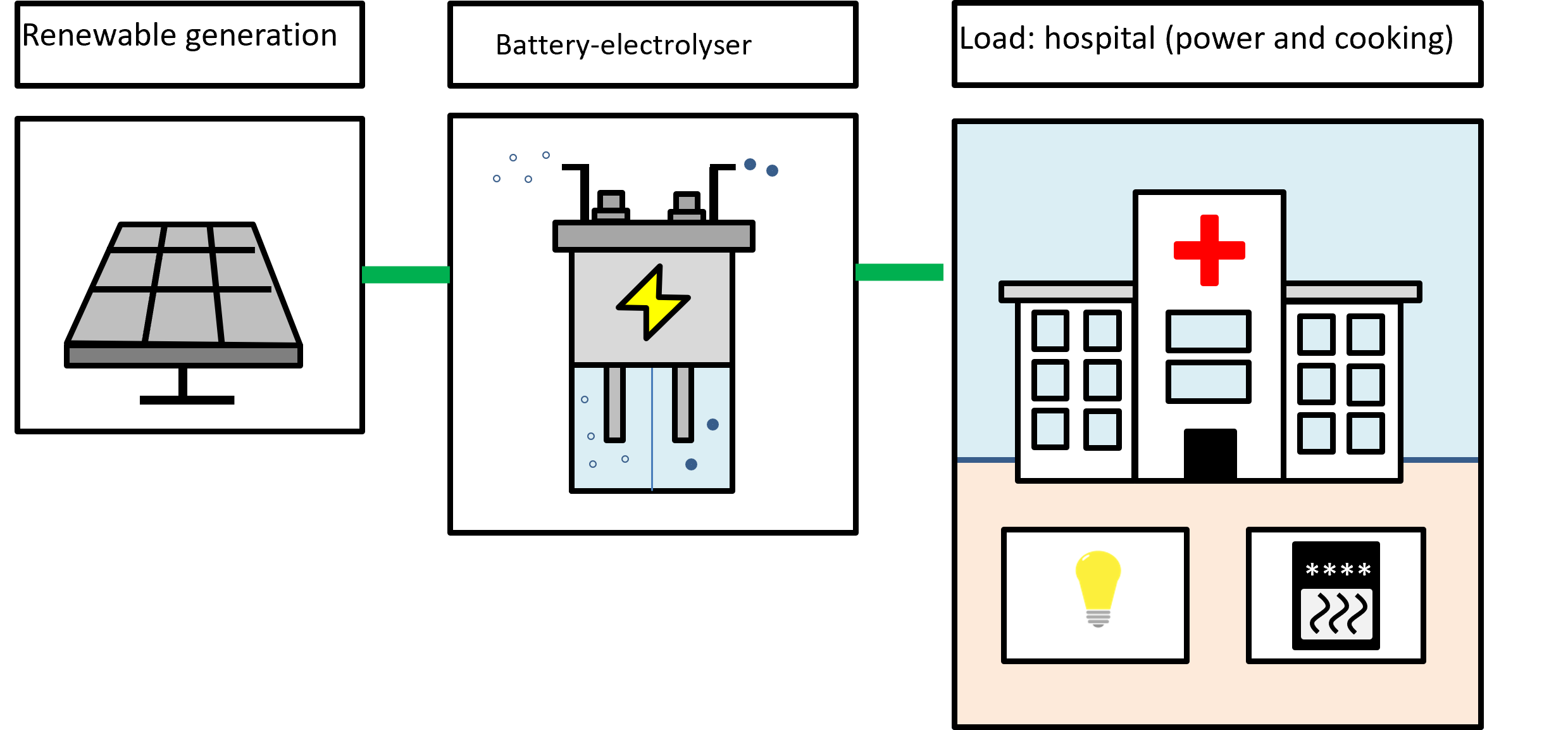
Battery-electrolysers in microgrids
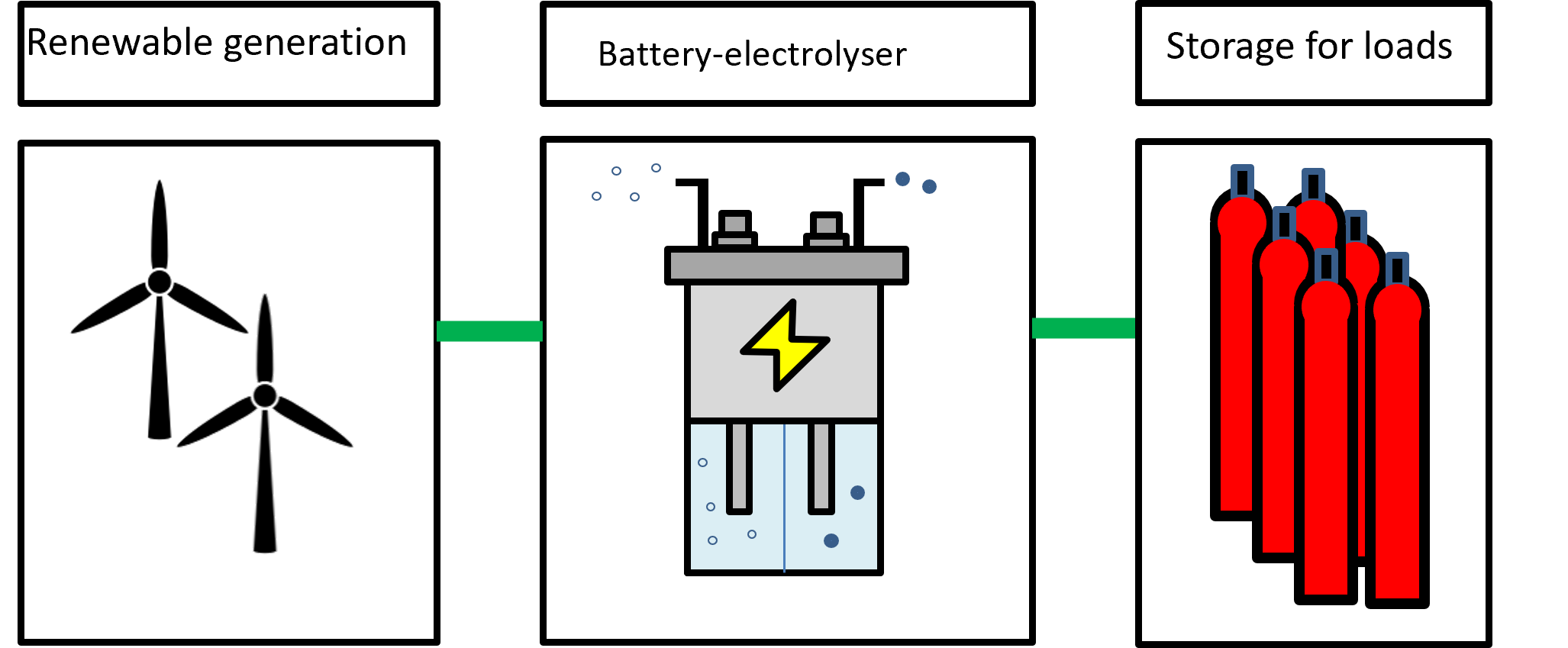
Battery-electrolysers using curtailed wind power
Our work includes, but is not limited to:
- Early TRL chemistry investigation
- Measurement and testing
- Modelling
- Prototyping
- Business case and use development