Marc grew up in Tea Tree Gully in Adelaide (South Australia) and did his undergraduate at the University of Adelaide majoring chemistry and genetics. After obtaining his Honours degree in 1994 with Dr. George Gream, investigating solvolysis routes to longifolene, he stayed at University of Adelaide for his postgraduate studies and obtained his PhD in chemistry in mid-1998 with Prof. Stephen F. Lincoln and A/Prof. A. David Ward on a thesis entitled ‘Synthesis and Physical Chemistry of Zinquin analogues’. In 1998, he moved to the University of Sydney as a Postdoctoral Fellow working on streptonigrin analogues in the group Prof. Margaret M. Harding, and then returned to the University of Adelaide in 2000 to work on the chemistry of endoperoxides with Prof. Dennis K. Taylor. In 2002 he relocated to the United Kingdom, taking up a PDRA position with Prof. J. Stephen Clark at the University of Nottingham where he completed the total synthesis of the F-J fragment of the polycyclic ether gambieric acid A. In late 2004 he joined Evotec as a medicinal Senior Scientist, but returned to academia in May 2006 where he took up a PDRA position with Prof. Christopher J. Moody at the University of Nottingham where he worked on synthetic routes to the antibiotic nosiheptide. In September 2008 he was appointed to his first independent academic position as a Lecturer in Organic Chemistry at Loughborough, and he was promoted to Senior Lecturer in 2015.
Research areas
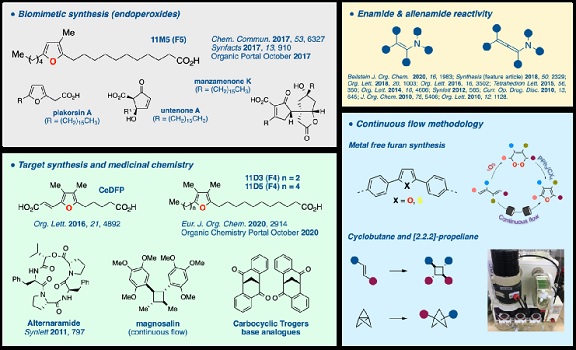
The recent research focus of the Kimber group has been on utilizing photochemical reactions in chemical synthesis.
The Kimber group has employed photoredox catalysis to explore the reactivity of electron rich functionalities such as enamides and their allene counterparts allenamides. We have published widely on the reactivity of allenamides and recently our group was the first to utilised photoredox catalysis to generate a key reactive intermediate used in allenamide chemistry. We have also employed this reactivity to provide expedient routes to novel heterocycles.
The biomimetic potential of photochemically generated singlet oxygen in natural product synthesis is also a source of great inspiration in our group. Using singlet oxygen together with the Kornblum DeLaMare rearrangement, synthetic routes to valuable furan fatty acids (FFAs) has been established. Furthermore, this avenue of research has provided valuable biomimetic insights on the possible origins of the FFAs; this is now being expanded to related polyketides.
We have invested in a commercially available continuous flow platform and we are currently translating and adapting some of our existing transformations to improve reaction efficiencies (metal free synthesis of functional materials). This platform is also being utilised to provide efficient methods to cyclobutane dimers and functionalised [2.2.2]-propellanes.
See an up to date list of Marc’s publications here; further details can be found on our external group webpage.
Current funded projects:
- Harnessing continuous flow for the synthesis of complex functional molecules. EPSRC DTA (project reference 2132426 related to EP/N509516/1 and EP/R51088/1).
- Development of odour additives for use in H2 technology. EPSRC CDT (project reference 2446456 related to EP/S023909/1).
Previous funded projects:
- Tackling Antimicrobial Resistance: An Interdisciplinary Approach. EPSRC 2015-08-31 to 2017-08-30 | grant (EP/M027341/1).
Available PhD positions:
Synthesis of Lamellarin alkaloids using a photoredox initiated cyclisation cascade
A biomimetic approach to peroxide-derived polyketide synthesis
Harnessing structural homology in polycyclic ether synthesis
Committee member of the Royal Society of Chemistry, Heterocyclic and Synthesis Group (2019 – present)
- Organising committee for the 24th RSC Lakeland Symposium (9th – 13th May 2019)
- Organising committee for the 25th RSC Lakeland Symposium (May 2021)
Committee member of the SCI/RSC Retrosynthesis competition (May 2019 – present)
- Organising committee for the 7th National SCI/RSC Retrosynthesis Competition (13th March 2020)
- Judge at the 7th National SCI/RSC Retrosynthesis Competition
- Organising committee for the 8th National SCI/RSC Retrosynthesis Competition (19th March 2021)
Member of the SCI Young Chemists’ Panel 2014-2016
- Member of the Royal Society of Chemistry.
- Member of the American Chemical Society.
- External examiner for PhD examinations in UK and overseas.
- Reviewer for the Czech Science Foundation.
- Referee for Journal of Organic Chemistry, European Journal of Organic Chemistry, Organic Letters, Synthesis, ACS Catalysis, RSC Advances, Beilstein Journal of Organic Chemistry, Chemical Science, Chemical Reviews.
- Research talks at the following meetings: 19th ICOS / RACI, Melbourne, Aust., July 2012; Young Chemists Symposia (YC12), Imperial College, UK, March 2012: 23rd ICHC, Glasgow, UK, August 2011: RSC East Midlands meeting, University of Leicester, UK, April 2010.
- Research seminars at the following institutions: Uni. of York, UK, 20th November 2013; Uni. of Glasgow, UK, 15th November, 2013; UCL, UK, 14th March 2012; Uni. of Sydney, Aust., 22nd February 2012; Uni. of Bern, Switzerland, 18th November 2011.
School responsibilities:
Departmental responsibilities
Programme Director – Medicinal and Pharmaceutical Chemistry (MPC)
Teaching responsibilities
- CMA102: Fundamental Synthetic Chemistry
- CMB102: Laboratory and Skills 2 (Chemistry)
- CMB103: Laboratory and Skills 2 (MPC)
- CMB112: Laboratory Skills for Natural Scientists
- CMC001: Modern Aspects of Organic Chemistry
- CMC026: Investigative Projects
- CMC027: Investigative Projects for Medicinal Chemists (module leader)
- CMD001: Research projects
- CMD401: Drugs: Modes of Action and Screening
- CMP056: Research Training Project
- CMP060: Drug Targets, Drug Design and Drug Synthesis
- CMP063: Professional Skills and Dissertation
