An international team of researchers has found a way to avoid the established principle that particles of the same charge repel each other – and opposite charges attract.
Charged atoms or molecules (ions) normally take on what is called Coulombic ordering where they sequence themselves in positive and negative succession along a straight line.
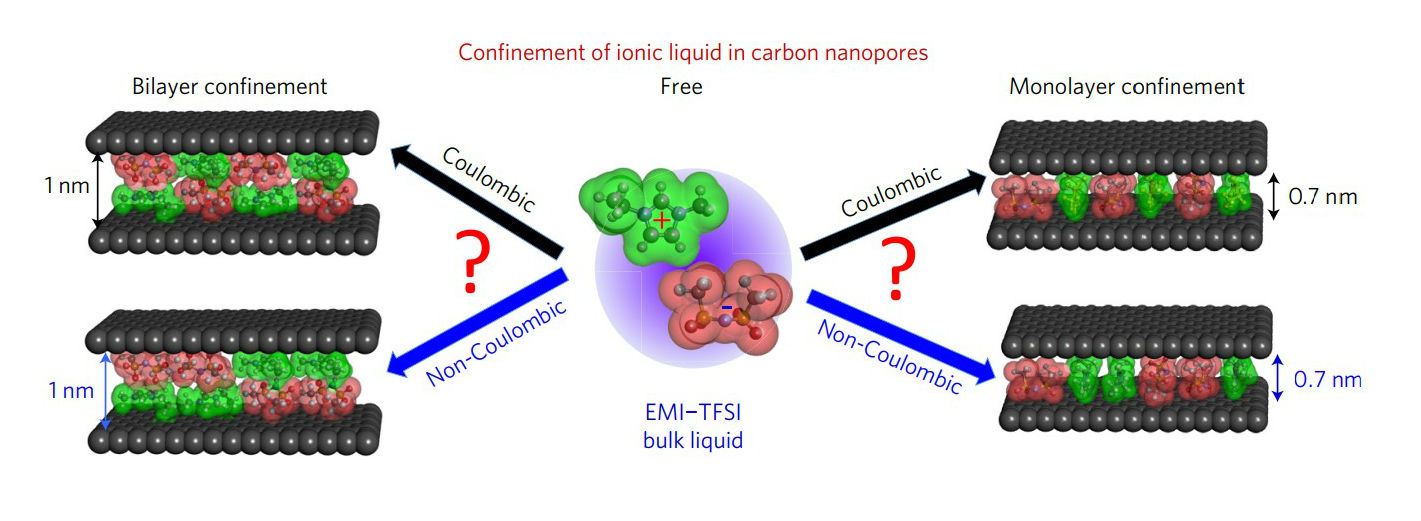
However, in a new paper, ‘Partial breaking of the Coulombic ordering of ionic liquids confined in carbon nanopores', the team shows how this normal Coulombic ordering starts to break down when ions are confined in miniscule carbon pores, less than a nanometer in diameter.
Disrupting the natural order of charged particles is important for the development of electrical energy storage devices such as batteries and supercapacitors, and could be pivotal for water treatment and alternative energy production technologies – which all involve ions packed into nanoporous materials.
Loughborough University’s Professor Mark Biggs, Dean of School of Science and Professor of Interfacial Science and Engineering, was part of the team and played a vital role in the 3D molecular modelling work that was central to interpreting the experimental data that led to the discovery.
He said: “Using 3D molecular models, we can determine from the experimental X-ray scattering data how the positively and negatively charged ions pack relative to each other inside the nanopores.
“Without this 3D molecular modelling ability, we would not have been able to discover the non-Coulombic packing.
“The primary driver for this work is electrical energy storage devices such as rechargeable batteries and supercapacitors.
“These devices are critical to making renewable energy available at any time, not just when the wind blows or the sun shines, and in developing the next generation of electrical vehicles.
“For example, supercapacitors are important for accelerating electrical cars from a standing start because they are capable of releasing their stored electrical energy very quickly in a way that batteries cannot.”
The breakthrough was made by an international team made up of scientists from Shinshu University, in Japan; Sabatier University, in Toulouse; Sorbonne University, in Paris; the French Research Network on Electrochemical Energy Storage, Drexel University, in Philadelphia, USA and the University of Adelaide, in Australia.
The discovery involved working with two carbon nanomaterials.One with pores at least a nanometer in diameter and another with pores less than a nanometer.
The researchers then adsorb in to both carbon materials some ionic liquid, which are room-temperature liquid salts often used as solvents in the chemical industry, as if they were a sponge sopping up water.
Normally the ions in ionic liquids arrange themselves in full compliance with the alternating positive-negative pattern of Coulombic ordering.
This was also observed for the ions that adsorbed into the carbon containing the larger pores.
However, for the other carbon where the pores are so small that only one layer of ions can be formed between the pore walls, negative ion pairs and positive ion pairs were found to widely occur.
“In this state, the Coulombic ordering of the liquid is broken,” the authors wrote. “Ions of the same charge neighbour each other due to a screening of their electrostatic interactions by the images charges induced in the carbon pore walls.”
The team observed this disruption in the natural order of ions through using molecular models to interpret x-ray scattering results.
They also reported that the non-Coulombic ordering became more pronounced when an electric charge was applied to the carbon material as would happen in charging and discharging electrical energy storage devices like supercapacitors.

“Our results suggest the existence of a molecular-scale mechanism that reduces the Coulombic repulsion energy between co-ions that become closer to each other,” they wrote.
This mechanism, they theorize, is linked to the charge temporarily induced in the walls of the carbon pores.
This “image charge in the pore walls”, they write, offsets the natural electrostatic repulsion of ions of the same charge within the pores, thus allowing the channels to fill with same-charged ions lined up next to each other.
Drexel University’s Professor Yury Gogotsi, one of the senior researchers involved in the work, said that the discovery brings scientists closer to using ionic liquids in batteries.
He said: “We can get safer batteries and supercapacitors when using ionic liquid electrolytes because the ionic liquids are not flammable like the electrolyte solutions currently used in these devices.
“Also, since there is no solvent, the entire volume is occupied by ions and we may be able to store more energy compared to conventional electrolytes that use organic solvents.”
The paper was published in Nature Materials this week.
ENDS

